The company's investments in independent research institutes in Basel, Switzerland, and Nutley, US, not only helped some of the world's most brilliant biomedical scientists succeed in Nobel Prizes but also paved the way for the discovery of entirely new classes of cancer drugs. In the 1980s, the world's first bioengineered cancer drug, interferon, was introduced; more recently, monoclonal antibodies developed by Roche and its partners have made cancer treatment more effective for many patients. Today, at a time when many companies are choosing to diversify, Roche's focus remains on innovation in pharmaceuticals and diagnostics.
A few key facts about Roche:
- Roche employs 101 200 people around the world.
- Roche generated annual revenue of $64.66 billion in 2020.
- The company spent almost 13 billion CHF on Research and Development in 2020, and with it, kept the unofficial title of highest R&D spender in pharmaceuticals.
- With 30 phase III projects and multiple projects currently in registration, Roche has one of the strongest drug development pipelines in the industry.
- Group sales have grown by 1%, driven by the 14% increase in diagnostics sales.
- A 2% decline in pharmaceutical sales is largely the result of biosimilars, especially in the US, and a reduction in hospitalizations and outpatient visits due to the COVID-19 pandemic.
- Several global restructuring plans initiated in prior years were implemented by the Group in 2020, which totaled almost 1 billion CHF.
Roche History
A 28-year old "pharmapreneur"
During the industrial revolution, Hoffmann-La Roche & Co. was founded in 1896. In Basel, Switzerland, Fritz Hoffmann-La Roche founded his company on October 1, 1896, when he was only 28 years old. As an early pioneer of pharmaceutical manufacturing, he recognized how useful it was to fight diseases.
Fritz Hoffmann-La Roche had a very ambitious plan: to produce and market drugs internationally in a single dose and with predictable effects. Despite initial setbacks and the threat of bankruptcy, he persevered with his vision. He soon expanded his company and launched products outside Switzerland before the end of the 19th century.
Between 1897 and 1910, the Grenzach factory in Germany was expanded and the majority of production took place there. Carl Meerwein and Fritz Hoffmann-La Roche wasted no time in setting up networks of agents and subsidiaries in Europe and overseas. The Roche company became truly international until 1914, opening offices in Milan, New York, St. Petersburg, and London.

Continuously developing the product portfolio
Vitamin preparations and derivatives were among the company's earliest products, including:
- The company produced a nonprescription cough syrup that contained its active substance, Thiokol. Almost immediately, orange-flavored syrup proved to be a success. The Sirolin syrup was introduced in 1898 and was on sale for 60 years.
- In collaboration with Max Cloetta, Roche developed a standardized and pure product that contains all the cardiac glycosides found in the purple foxglove leaf. No unnecessary ingredients were used and the preparation was uniformly effective. The Digalen preparation was introduced by Roche in 1904. Digalen stayed on the market until 1964.
- The analgesic Pantopon was launched in 1909 as a result of joint research with Roche's partners and extensive studies.
By 1914, Roche employed over 700 people around the globe at multiple locations. As part of its expansion efforts, Roche aimed to create strong collaborative relations between academic circles. Until 1915, all Roche innovations were the result of such partnerships.
The company suffered heavy financial losses as a result of the First World War. The boycott in Germany, the complete closure of the Russian market, and the strong currency fluctuations caused by the war shook the Group, which had been a solid Swiss company until then. In addition, Roche mourned the passing of Fritz Hoffmann, the project's visionary founding father. The company was pulled out of its downward spiral by research to introduce a new product line.
In 1920, Markus Guggenheim published a classic study of biogenic amines. This laid the foundation for the launch of a new product line, biochemicals, including amino acids, peptides, proteins, cardiac glycosides, vitamins, and hormones. Roche's biochemicals improve its reputation among scientists.

Guggenheim also studied vitamin B1. At Roche, he worked with extracts of rice bran to synthesize and produce this vitamin.
Other products were also created in the following decades, including:
- The company produced synthetic vitamin C for the first time under the brand name Redoxon in 1934. Roche became the world's leading producer of vitamin A, B1, B2, E, and K1. Almost all of the company's products were vitamins by 1938.
- In 1956, the first antidepressant, iproniazid, was accidentally created during an experiment while synthesizing another active ingredient. The purpose of the research was to develop a better drug for treating Tuberculosis.
- Hoffmann-La Roche & Co. introduced the benzodiazepine class of tranquilizers in 1957 (of which Valium and Rohypnol are the best known).
Pioneering in gender equality
Roche was among the first companies to appoint a woman in senior management. After obtaining a Ph.D. in political economy, Alice Keller worked at Roche Basel for about a year before accepting a position with its Japanese subsidiary in 1925. Her first job was administrative assistant, dealing with correspondence, revising documents, and helping with billing and costing. Keller had advanced to the status of Direktorin by the time she returned in 1939, which was an unprecedented accomplishment at the time.
Becoming market leader
Biomedical Reference Laboratories was acquired by Roche’s US company in 1982 for US$163.5 million. This company dates back to the late 1960s and was situated in North Carolina. After the merger, Hoffmann-La Roche incorporated the combined company as Roche Biomedical Laboratories, Inc. in Burlington. In the early 1990s, Roche Biomedical had grown into one of the United States' largest laboratory systems. It had 20 major laboratories and sold US$600 million.
Genentech was acquired by Roche for $46.8 billion in 2009. Genentech, in which Roche held a majority stake since 1990, was fully acquired by Roche after eight months of negotiations. The Genentech acquisition led Roche to relocate its Palo Alto-based research facilities on the Genentech campus in South San Francisco, while its headquarters in New Jersey, located on the site since 1929, moved to Genentech's facility in Clifton, New Jersey.
As of today, the Roche Group merged with 50 companies, including InterMune ($8.90B in2014) and Spark Therapeutics ($4.30B in 2019). Roche’s latest M&A deal (September 2021) was TIB MOLBIOL, a company providing custom synthesis of oligonucleotides - eventually enhancing Roche’s molecular diagnostics operations.
Key takeaway:
From the beginning, Roche's success has been based on the instincts of its founders, who supported countless research projects that resulted in products that have been used for decades. Roche's history has not been without setbacks, but the company has also achieved significant results on a historic scale thanks to a consistently substantial research and development budget.
Products such as synthetically produced vitamins, antidepressants, and later tests (e.g. for HIV) have helped the company achieve great success in many areas of medicine.
Initially, the company's expansion strategy was based on making sales and manufacturing processes cheaper, but by the 1900s it was acquiring smaller and larger pharmaceutical companies every two years on average to continually expand its portfolio. Today, internal R&D programs and the use of external expertise are clearly the drivers of the expansion.
Different divisions of the company

Roche’s pharmacological division
Roche mainly offers drugs for oncology, immunology, hematology, central nervous system disorders, and certain rare diseases. The number of therapeutic areas in which Roche products are used has increased steadily in recent years. Roche products are used in many hospitals globally, and these medicines are particularly important for the treatment of oncology patients.
As an ultimate goal, the company wants to make personalized medicine widely available to patients, and since the launch of the FoundationOne program, it has given hundreds of patients the option of personalized therapy based on gene sequencing. Using FoundationOne Liquid CDx, treatment decisions for solid tumor cancer can be influenced by the analysis of more than 300 cancer-related genes. The necessary insights can be gathered through blood draw, instead of a highly invasive and much more expensive biopsy procedure.
Diagnostics division
The Roche Diagnostics Division is a global leader in in-vitro diagnostics (IVD). Our tests are used by hospitals, commercial laboratories, physicians, and patients to diagnose diseases and genetic mutations, select appropriate therapies and monitor patient response to treatment using blood, tissue, and other samples.
In-vitro diagnostics has long been the gray area of healthcare, accounting for more than 60% of clinical decisions and only 2% of total healthcare spending. Roche employees work with laboratory partners worldwide to increase the speed, accuracy, and reliability of laboratory testing through workflow automation, integration, and information management. This collaboration has enabled laboratories to better manage the challenges of increasing numbers of tests and data. This has led, for example, to tests used for screening, diagnosis, prognosis, and prediction of disease progression.
Roche is also developing diagnostic tools that help select the right treatment for a given drug and predict response to treatment. Every year, billions of diagnostic tests are performed in various laboratories using Roche instruments. Roche is the market leader in both public and private laboratories.
Roche’s IVD products are offered in the following market segments:
- Centralized and Point of Care Solutions
- CustomBiotech
- Diabetes Care
- Diagnostics Information Solutions
- Molecular Diagnostics & Research
- Sequencing
- Solution Integration and Services
- Tissue Diagnostics & Research
- Hospital & Laboratory Analytics
Diabetes division
The diabetes division of Roche has been leading the way in diabetes management technology and services for 40 years. The division's more than 5,000 employees work in around 100 markets worldwide to make diabetes management even easier and more commonplace. The company not only develops and manufactures essential devices for people with diabetes but also offers integrated solutions that enable fully personalized patient care.
Diabetes is now one of the most common chronic diseases worldwide and is considered a widespread disease. Diabetes affects more than 33 million Europeans. A study by the International Diabetes Federation (IDF) predicts that there will be approximately 38 million diabetics in the EU by 2030. A similarly alarming rate can be observed in the US: As of 2020, an estimated 34.2 million people have diabetes according to the National Diabetes Statistics Report from the CDC (Centers for Disease Control).
One of the business area's main goals is the early detection of diabetes and modern therapy to prevent serious diabetes-related complications. The business area's main products are blood glucose meters for patients for home use, associated test strips, finger-prick tests, and electronic and paper-based blood glucose monitoring systems. These devices provide accurate and reliable results in a few seconds using a tiny drop of blood.
The company's insulin pumps, associated infusion devices, software, and other accessories, and related services (patient education programs and comprehensive customer support) provide comprehensive therapeutic solutions to improve the quality of life of patients requiring insulin therapy.
Shared service centers around the world
Like most large international companies, Roche centralized processes that can be performed in a well-standardized manner regardless of location. Typical functions are IT, finance and accounting, telemarketing, helpdesk, and customer service, which are usually organized in shared service centers.
The idea of SSC is to break down the processes of a particular function, such as the IT helpdesk, into transparent sub-elements and then divide up the tasks so that they can be performed as simply as possible and link performance indicators to them.
SSCs are usually set up in countries where the workforce still has the appropriate skills but is significantly cheaper than in industrialized countries. Roche has three shared service centers in Kuala Lumpur (Malaysia), San Jose (Costa Rica), and Budapest (Hungary). In the latter case, labor is more expensive than at the other two sites, but the Hungarian SSC is entrusted with more complex tasks.
Roche Service (Europe) Ltd. was founded in 2006 as a subsidiary of the Roche Group in Budapest. It provides global support and professional advice to Roche's more than 120 subsidiaries in the areas of finance, procurement, human resources, and IT.
Its central location in Europe, extensive language skills, and high quality of education made Hungary an attractive location for Roche. In the year of its establishment, a team of 20 people started working in the spirit of the company's global values, followed by steady growth. The service center is currently expanding and developing new and increasingly complex services. The gradual and continuous improvement of processes and the increase in the number of end-to-end services provided by the service center have led to the increasing complexity of tasks.
Key takeaways

As a responsible stock exchange-listed company, Roche invests a lot of energy in transparency so that we know exactly which business areas the company is divided into and what it does.
Although the pharmaceuticals business, Roche's main source of revenue, is currently in decline (mainly due to the difficulties caused by the COVID-19 pandemic), it has generated a significant portion of the company's sales since its inception.
The other main business, in-vitro diagnostics, which came later, is currently growing at a tremendous rate and could become the company's main source of revenue in the long term. While COVID has weighed on the pharma division, it has boosted the IVD division, as Roche has been able to further extend its market leadership in this area by developing COVID tests.
One of the greatest challenges of our time is the fight against diabetes, which affects Roche's activities in several areas. However, given the strategic importance of this disease, Roche has organized this quasi-vertical product range into a completely separate business area. Diabetes medicines, measuring and testing equipment, and diagnostic instruments for laboratories are all part of this business area, which is generating increased sales.
Like other multinationals, Roche is taking advantage of shared service centers. The company has outsourced its functions such as IT helpdesk, HR, finance, and purchasing to three sites where it can find suitably qualified personnel at significantly lower wages than in Western Europe. The functions provide Roche subsidiaries with increasingly complex services that are as fully documented and transparent as possible. The SSCs generally operate according to strict KPIs, which are one of the benchmarks by which they demonstrate continuous improvements and financial savings to management.
Roche And Its Subsidiaries
Roche Holding AG
The Roche Group consists of more than 120 subsidiaries, including the parent company's local technical companies, more than 50 companies that have been incorporated into the Group, and companies that operate shared service centers. The Group is united under one umbrella organization, Roche Holding AG.
Genentech

In March 2009, Genentech joined the Roche Group. The United States pharmaceutical operations of Roche and Genentech were combined by their merger agreement. Currently, Roche pharmaceutical operations in the United States are housed at Genentech's South San Francisco campus. Gentech Research and Early Development operates as a separate entity within the Group.
Herbert Boyer and venture capitalist Robert A. Swanson founded the company in 1976. Only one year later, they were the first to successfully express a human gene in bacteria and also created synthetic human insulin shortly afterward. A majority stake in Genentech was acquired by Hoffmann-La Roche AG in 1990. Roche acquired the company in March 2009 for approximately $46.8 billion by purchasing the remaining shares. Genentech has also done two acquisitions.
Ventana Medical Systems

Dr. Thomas Grogan founded Ventana Medical Systems, Inc. in 1985. The company rejected a hostile takeover bid of $75 a share from Roche Holding AG in 2007. The final and successful cash offer for Ventana was $89.50 ($3.4 billion) in 2008.
By automating the testing process, the Roche Tissue Diagnostics team has revolutionized cancer diagnostics. With more than 250 tests available, it is among the leading global providers of cancer diagnostic systems.
Through tests, Roche TD can determine which patients will benefit most from a particular therapy. Their companion diagnostics link patients with the most appropriate and targeted treatment options. Roche partnered with the company to develop diagnostics that give patients personalized treatment options, including immunotherapies that stimulate the body's immune system to kill cancer cells.
A monoclonal antibody developer, Spring BioScience Corp, was Ventana's first independent acquisition in 2007.
Chugai Pharmaceutical Co.
A strategic alliance with Roche was completed in 2002, which allows Chugai to become a member of the Group. A majority of Chugai's stock (62%) was acquired by Roche in October 2002.
Pharmaceutical company Chugai Pharmaceutical Co. is based in Tokyo, Japan. Chugai retained its management autonomy and its company name was not changed during this merger. The Japanese company has since been listed on the website Tokyo Stock Exchange, as it was an important point of agreement in the merger agreement.
By joining Roche, Chugai gained exclusive global marketing rights for Roche products in Japan. Chugai is also gaining access to Roche's global market for its brand of products by licensing them out.
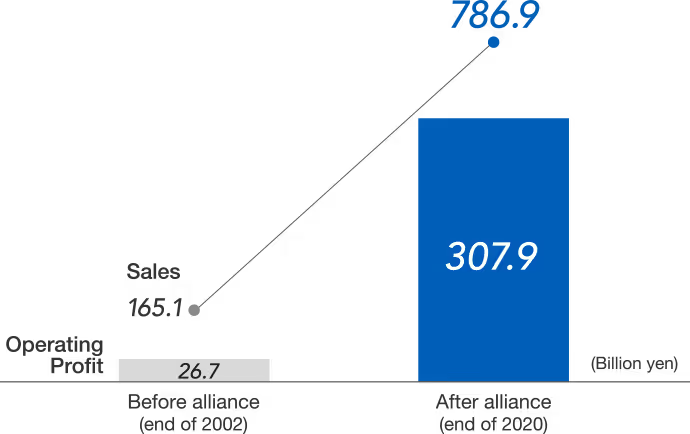
Chugai has developed world-class antibody engineering technologies with the stable revenue generated through its strategic alliance with Roche, as well as strives to develop capabilities for middle molecules thanks to the stable revenue gained through the alliance. Several of Chugai's discoveries have also been recognized by the US Food and Drug Administration (FDA).
Other key partnerships
The key growth driver at Roche is partnerships, which, based on the Group’s 2020 annual report, have resulted in the following innovations in 2020:
- Blueprint Medicines/Roche: A license agreement and long-term collaboration with BM to help patients with different types of cancer.
- Reverie Labs/Roche/Genentech: AI is a new element in drug discovery processes, which will empower Roche Group to further enhance its product design and innovation capabilities. With Reverie's machine learning platform, drug discovery will be augmented and accelerated by increasing the predictive power of algorithms for relevant small-molecule properties. Roche's pRED and gRED platforms will use this new technology to enable the delivery of twice as many medical advances at half the cost.
- Arrakis/Roche: Development of RNA-targeted small molecules and a licensing agreement.
- Vividion/Roche: With Vividion's proprietary drug discovery platform, pRED can pursue therapeutics across multiple oncology and immunology targets.
- Besides developing new drug discovery technologies, Roche also formed new partnerships spanning a wide range of therapeutic areas and modalities.
Key takeaways
The involvement of external resources has been an important element of the Roche Group's expansion strategy from the very beginning. While in the early days close partnerships were typically entered into only at the level of individual researchers or laboratories, the Roche Group later acquired entire companies to gain access to valuable know-how, products, markets, and other opportunities.
Currently, the three companies mentioned above form Roche's group of major subsidiaries, but this may change in the future through ongoing acquisitions.
R&D and the company’s other values
R&D as the main driver of growth

Since the company’s founding, Roche has always sought to leverage its well-established academic relationships to build partnerships for new products. Today, Roche's research and development division is a multi-billion-dollar business that continues to achieve historic results in the pharmaceutical industry thanks to its exceptionally high budget.
It is Roche's powerful advantage to combine diagnostics and pharmaceuticals in one company, as today’s personalized healthcare requires close coordination between these two groups. The two divisions work together on projects regardless of how their R&D processes differ. They share expertise, facilities, and technologies.
Furthermore, the company believes diversity of scientific opinion will lead to the innovations in healthcare that society so desperately needs. The company thus maintains three pharmacies and more than 150 external partners, all of which are independently operated.
Pharma Research and Early Development (pRED), Genentech Research, and Early Development (gRED), and the Japanese Chugai are the three most important divisions of Roche’s R&D leg. With a vision to redefine what is possible in healthcare, the Diagnostics Division is a healthcare pioneer and the leader in in-vitro diagnostics. Development of new diagnostic testing, laboratory efficiency, healthcare digitization, and decision support tools and software is the ultimate goal of this division.
The Roche Diagnostics Business Areas include:
- Centralized and Point of Care Solutions (CPS)
- Molecular Diagnostics
- Tissue Diagnostics
- Sequencing
- Diagnostics Information Solutions (DIS)
- Diabetes Care.
The division has R&D facilities in the United States, Austria, Germany, Poland, Switzerland, South Africa, and China.
Pharma Research and Early Development (pRED)
Pharma Research and Early Development (Roche pRED) is one of four divisions within the Roche Group. The range of research topics are covered by the Discovery & Translational Medicine Areas (DTAs) at Roche pRED focusing on immunology, infectious diseases and ophthalmology, neuroscience and rare diseases; and oncology. More than 2,200 scientists from throughout the world are part of the pRED internal staff and the broader community.
Roche's expenditure on research and development from 2011 to 2020

The process of R&D at Roche
The following figure clearly shows that the innovation processes at Roche do not differ significantly from those of other pharmaceutical companies. The first step in the R&D process is the discovery phase, which - and this is not clear from the diagram - can take decades. During this time, researchers search for solutions in a project system to achieve the set goal. Once the theoretical solutions are developed and validated, the experimental phase follows with a fixed project budget. Most drug development projects are discontinued at this stage because they do not always succeed in translating theory into practice. However, if the researchers succeed in finding a reproducible solution, the trials can begin and the approval process can be completed.
Key takeaways
The Group's main driving force is clearly the constant quest for innovation, the need to develop as many new medicines and therapeutic solutions as possible under the Roche umbrella. Roche holds nearly 300 important patents, and many of its medicines are either approved or under review by the FDA.
The company spends a significant portion of its annual revenue on research and development, for example, more than 13 billion Swiss francs in 2020, which is exceptionally high even in the world of pharmaceuticals. In addition to the Group's continuous expansion through mergers and acquisitions, research and development are the growth engine.
Other Growth Driving Factors
Production
The COVID-19 pandemic era brought great potential for the medicine industry, and Roche had to respond immediately. Although drug sales were lower than expected, the Group was able to compensate with its diagnostics division, which produced millions of COVID tests.
Roche Molecular Systems' Branchburg team worked around the clock to deliver millions of tests per month. To enhance production capacity while maintaining manufacturing workers' health and safety, multiple task forces were formed. In 2020, PCR output was multiplied by four.
As of 2017, Roche had 21 locations for manufacturing, spread over almost all of the habitable continents (excluding Australia).
Company values as part of the strategy
Roche’s business priorities:
- Patients should be the priority
- Advancing science through excellence
- A personalized approach to healthcare
- Healthcare accessibility
- An excellent work environment
- Sustainability
Sales and marketing
Key opinion leaders, partners, external agencies, and the press depend on Roche Marketing and Sales to effectively communicate the patient benefits of its medicines and diagnostic system solutions.
A sales team's responsibilities include acquiring customers, caring for them, conducting market research, analyzing the market, identifying new trends, and monitoring the competition. The sales team of Roche attends trade fairs and congresses, presents sales presentations to medical specialists and health experts, and advises healthcare professionals on the proper use of our products.
Pharma and Diagnostics rely on marketing for strategic business input and guidance to achieve their business goals. To recognize and meet global market needs, the marketing department also collaborates with affiliate markets.
Logistics, and technology transfer
With pharmaceutical products, not only is the manufacturing process complex but the associated storage and transport tasks also require special expertise. Roche works with several partners to ensure that its products reach their destination by land, sea, and air.
In addition to Roche products, there is also the extraordinary need to transport technology. This is what happened in 2020 when Genentech experts carried out a technology transfer at the decision of the management board. It required them to transfer skills, knowledge, technologies, and methods needed to produce specific products and processes from Roche production sites to others. Technology transfers typically take between 12 and 18 months. The Hillsboro team completed the process without compromising safety or quality in just 4 weeks.
Supply chain management
Investing in business and supplier partnerships will make Roche's manufacturing procurement organization more effective at bringing innovative solutions to patients more quickly and efficiently.
Roche's overall business objectives align with those of its supply chain. Below is a list of key 2020 targets for pharmaceutical manufacturers:
- Supply On-Time-In-Full: maintain >97%
- Close to zero out-of-stock medicines
- Cut energy, water use, and Roche K6 Directive topics by 2% compared to 2019 levels
- Leading the way in manufacturing, quality analysis, and sales release lead times: Top quartile in the benchmark for pharma
- For 80% of sales, the lead time for finished goods is 30 days
Key takeaways
Roche is a pioneer in sales, marketing, logistics, supply chain, and manufacturing, in addition to the two key growth drivers.
The Group's manufacturing operations are diversified, with large production units on almost every continent, the largest are located at its headquarters in Switzerland and the factories of its U.S. and Japanese subsidiaries.
In sales and marketing, Roche employees use both inbound and outbound solutions, with the main focus on strengthening and maintaining Roche's position as a scientific leader. The two main keywords, brand and reputation, aim in the same direction.
Among the logistical tasks, the various technology transfer tasks have recently emerged. With the acquisition of new companies, the need to quickly transfer know-how and technology between the various existing and new sites of the Group regularly arises.
The Group mobilizes considerable resources to review and monitor its more than 60,000 suppliers and implement a KPI system rigorous enough to ensure high quality.
Final thoughts and key takeaways of Roche’s story
Growth by numbers
It is not entirely straightforward that a pandemic represents an exclusively positive return for a healthcare group, as Roche has also suffered losses in some areas despite its steady growth in recent years. At the same time, the group's diagnostics business is expanding at an unprecedented pace, and the relative weakness of its pharmaceuticals business is being offset by the company's M&A activity.
For the years 2015 to 2020, the Group has a clear development path resulting from continued diversification and leadership in healthcare innovation with the highest R&D budget in the industry.
Key takeaways from Roche’s story:
- Strong academic ties: From the beginning, the Group has sought to channel external knowledge into product development and has funded a number of research and academic rounds that it expects will lead to the invention of marketed products, primarily pharmaceuticals.
- Internal structure: The diversified corporate structure is designed to meet the interests of Roche's various business areas. For example, the Diabetes Division is completely separate, although its activities span several areas that overlap with other divisions.
- Shared service centers: Roche uses the shared service center model for two reasons: to take advantage of cheaper labor and to centralize knowledge in a way that makes processes faster and clearer.
- M&A: Mergers and acquisitions have been part of the Group's strategy since the 1980s, and in the more than 40 years since, 50 transactions have been successfully completed. By merging new companies (even if some companies have remained completely independent), Roche gains products, markets, know-how, patents, and experienced employees.
Production at 21 locations, pioneering healthcare sales and marketing, complex logistics, and supply chain solutions complete Roche's growth story of 125 years. During this time, history and economic life have presented the company with countless challenges, but it has so far successfully overcome them all thanks to its strong growth engine, the support of research and development. If there is one thing we as entrepreneurs take away from this story, it should be the importance of investing in knowledge, which skyrocketed Roche to become a market leader.



