A clinical trial timeline plan is a strategic roadmap that outlines the key stages of a clinical trial and the timeframe in which each stage should be completed. It helps pharmaceutical and healthcare organizations, clinical research teams, and project managers to track the progress of a clinical trial and ensure that all tasks and milestones are completed in a timely manner. The plan also helps ensure that all regulatory requirements are met and that data accuracy and integrity are maintained throughout the trial.
Each focus area has its own objectives, projects, and KPIs to ensure that the strategy is comprehensive and effective.
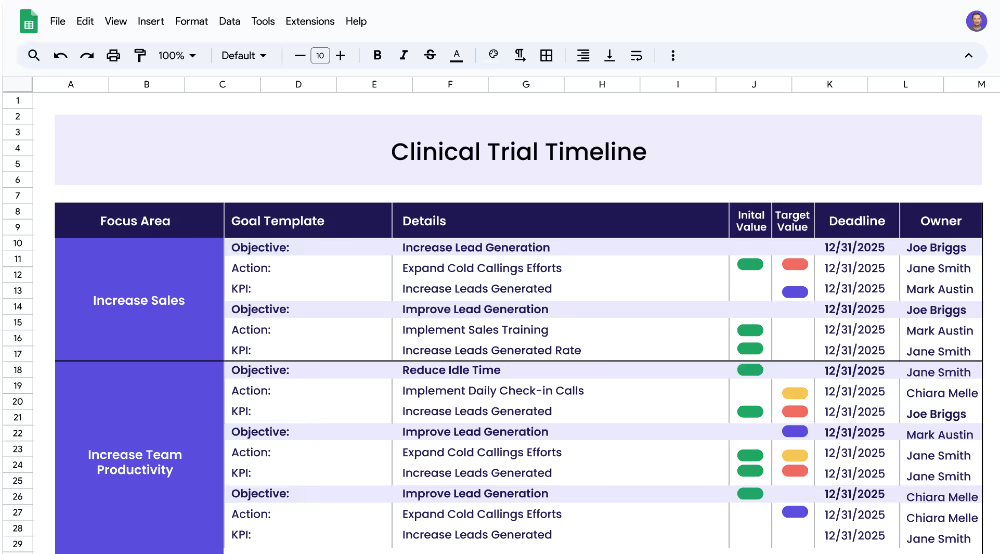
This clinical trial timeline plan template is designed to help pharmaceutical and healthcare organizations, clinical research teams, and project managers to create a strategic roadmap and timeline for their clinical trials. The template outlines the focus areas, objectives, measurable targets (KPIs), and related projects necessary for a successful clinical trial and can be used to track progress and ensure that all tasks and milestones are met on time.
A focus area is a broad area of interest or concern that forms the basis of a clinical trial. Examples of focus areas in clinical trials include clinical trial timeline, data management, and regulatory requirements. Each focus area should be broken down into objectives and related projects in order to ensure successful completion of the clinical trial.
Objectives are specific goals or outcomes that should be achieved as part of a clinical trial. Objectives should be measurable and have a clear target. Examples of objectives could include establishing a timeline for a clinical trial, ensuring tasks and milestones are met on the timeline, monitoring data accuracy and integrity, and ensuring documents are submitted in a timely manner.
Key performance indicators (KPIs) are measurable targets that should be set in order to achieve a specific objective. KPIs should be monitored and tracked throughout the clinical trial. Examples of KPIs could include decreasing the timeline from 12 months to 8 months, increasing the on-time completion rate from 50% to 80%, increasing data accuracy from 80% to 95%, and decreasing data collection time from 3 days to 1 day.
Projects (or Actions) are the initiatives that need to be completed in order to achieve the KPIs. Examples of projects could include developing a timeline of clinical trial stages, creating a project plan to track tasks and milestones, implementing a data monitoring system, creating a data collection plan, and implementing a regulatory compliance system.
If you’re ready to accelerate your strategy and see quicker results, Cascade Strategy Execution Software is your next step. Unlike traditional spreadsheets that slow down your process, Cascade provides a dynamic platform that enhances real-time updates, centralized collaboration, and automated reporting. This means your team can easily track progress, adjust strategies quickly, and maintain alignment across all levels. Sign-up for free or book a demo with one of our strategy experts to explore how Cascade can streamline your strategic process and beyond.